Streptococcus pyogenes (Group A streptococcus) is a Gram-positive, nonmotile, nonsporeforming coccus that occurs in chains or in pairs of cells. Individual cells are round-to-ovoid cocci, 0.6-1.0 micrometer in diameter (Figure 1). Streptococci divide in one plane and thus occur in pairs or (especially in liquid media or clinical material) in chains of varying lengths. The metabolism ofS. pyogenes is fermentative; the organism is a catalase-negative aerotolerant anaerobe (facultative anaerobe), and requires enriched medium containing blood in order to grow. Group A streptococci typically have a capsule composed of hyaluronic acid and exhibit beta (clear) hemolysis on blood agar.
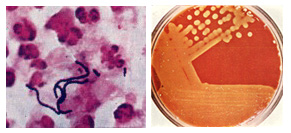
Figure 1. Streptococcus pyogenes. Left. Gram stain of Streptococcus pyogenes in a clinical specimen. Right. Colonies of Streptococcus pyogenes on blood agar exhibiting beta (clear) hemolysis.
Streptococcus pyogenes is one of the most frequent pathogens of humans. It is estimated that between 5-15% of normal individuals harbor the bacterium, usually in the respiratory tract, without signs of disease. As normal flora, S. pyogenes can infect when defenses are compromised or when the organisms are able to penetrate the constitutive defenses. When the bacteria are introduced or transmitted to vulnerable tissues, a variety of types of suppurative infectionscan occur.
In the last century, infections by S. pyogenes claimed many lives especially since the organism was the most important cause of puerperal fever (sepsis after childbirth). Scarlet fever was formerly a severe complication of streptococcal infection, but now, because of antibiotic therapy, it is little more than streptococcal pharyngitis accompanied by rash. Similarly, erysipelas (a form of cellulitis accompanied by fever and systemic toxicity) is less common today. However, there has been a recent increase in variety, severity andsequelae of Streptococcus pyogenes infections, and a resurgence of severe invasive infections, prompting descriptions of "flesh eating bacteria" in the news media. A complete explanation for the decline and resurgence is not known. Today, the pathogen is of major concern because of the occasional cases of rapidly progressive disease and because of the small risk of serious sequelae in untreated infections. These diseases remain a major worldwide health concern, and effort is being directed toward clarifying the risk and mechanisms of these sequelae and identifying rheumatogenic and nephritogenic strains of streptococci.
Acute Streptococcus pyogenes infections may present as pharyngitis (strep throat), scarlet fever (rash), impetigo (infection of the superficial layers of the skin) or cellulitis (infection of the deep layers of the skin). Invasive, toxigenic infections can result in necrotizing fasciitis, myositis and streptococcal toxic shock syndrome. Patients may also develop immune-mediated post-streptococcal sequelae, such as acute rheumatic fever and acuteglomerulonephritis, following acute infections caused by Streptococcus pyogenes.
Streptococcus pyogenes produces a wide array of virulence factors and a very large number of diseases. Virulence factors of Group A streptococci include: (1)M protein, fibronectin-binding protein (Protein F) and lipoteichoic acid for adherence; (2) hyaluronic acid capsule as an immunological disguise and to inhibit phagocytosis; M-protein to inhibit phagocytosis (3) invasins such asstreptokinase, streptodornase (DNase B), hyaluronidase, andstreptolysins; (4) exotoxins, such as pyrogenic (erythrogenic) toxin which causes the rash of scarlet fever and systemic toxic shock syndrome.
Classification of Streptococci
Hemolysis on blood agar
The type of hemolytic reaction displayed on blood agar has long been used to classify the streptococci. Beta -hemolysis is associated with complete lysis of red cells surrounding the colony, whereas alpha-hemolysis is a partial or "green" hemolysis associated with reduction of red cell hemoglobin. Nonhemolytic colonies have been termed gamma-hemolytic. Hemolysis is affected by the species and age of red cells, as well as by other properties of the base medium. Group A streptococci are nearly always beta-hemolytic; related Group B can manifest alpha, beta or gamma hemolysis. Most strains ofS. pneumoniae are alpha-hemolytic but can cause ß-hemolysis during anaerobic incubation. Most of the oral streptococci and enterococci are non hemolytic. The property of hemolysis is not very reliable for the absolute identification of streptococci, but it is widely used in rapid screens for identification of S. pyogenes and S. pneumoniae.
Antigenic types

Figure 2. Cell surface structure of Streptococcus pyogenes and secreted products involved in virulence.
FIGURE 3. Pathogenesis of Streptococcus pyogenes infections. Adapted from Baron's Medical Microbiology Chapter 13, Streptococcus by Maria Jevitz Patterson.
The occurrence of cross-reactive antigens in S. pyogenes and heart tissues possibly explains the autoimmune responses that develop following some infections. The antibody mediated immune (AMI) response (i.e., level of serum antibody) is higher in patients with rheumatic fever than in patients with uncomplicated pharyngitis. In addition, cell-mediated immunity (CMI) seems to play a role in the pathology of acute rheumatic fever.
Acute glomerulonephritis results from deposition of antigen-antibody-complement complexes on the basement membrane of kidney glomeruli. The antigen may be streptococcal in origin or it may be a host tissue species with antigenic determinants similar to those of streptococcal antigen (cross-reactive epitopes for endocardium, sarcolemma, vascular smooth muscle). The incidence of acute glomerulonephritis in the United States is variable, perhaps due to cycling of nephritogenic strains, but it appears to be decreasing. Recurrences are uncommon, and prophylaxis following an initial attack is unnecessary.
In immune individuals, IgG antibodies reactive with M protein promote phagocytosis which results in killing of the organism. This is the major mechanism by which AMI is able to terminate Group A streptococcal infections.M protein vaccines are a major candidate for use against rheumatic fever, but certain M protein types cross-react antigenically with the heart and themselves may be responsible for rheumatic carditis. This risk of autoimmunity has prevented the use of Group A streptococcal vaccines. However, since the cross-reactive epitopes of the M-protein are now known, it appears that limited anti-streptococcal vaccines are on the horizon.

FIGURE 4. Phagocytosis of Streptococcus pyogenes by a macrophage.CELLS alive!
The hyaluronic acid capsule allows the organism to evade opsonization. The capsule is also an antigenic disguise that hides bacterial antigens and is non antigenic to the host. Actually, the hyaluronic acid outer surface of S. pyogenesis weakly antigenic, but it does not result in stimulation of protective immunity. The only protective immunity that results from infection by Group A streptococcus comes from the development of type-specific antibody to the M protein of the fimbriae, which protrude from the cell wall through the capsular structure. This antibody, which follows respiratory and skin infections, is persistent. Presumably, protective levels of specific IgA is produced in the respiratory secretions while protective levels of IgG are formed in the serum. Sometimes, intervention of an infection with effective antibiotic treatment precludes the development of this persistent antibody. This accounts, in part, for recurring infections in an individual by the same streptococcal strain. Antibody to the erythrogenic toxin involved in scarlet fever is also long lasting.
Table 1. Summary of virulence determinants of Streptococcus pyogenesAdherence (colonization) surface macromolecules
M protein
Lipoteichoic acid (LTA)
Protein F and Sfb (fibronectin-binding proteins)
Enhancement of spread in tissues
Hyaluronidase hydrolyses hyaluronic acid, part of the ground substance in host tissues.
Proteases
Streptokinase lyses fibrin
Evasion of phagocytosis
Capsule: hyaluronic acid is produced.
C5a peptidase: C5a enhances chemotaxis of phagocytes .
M protein is a fibrillar surface protein. Its distal end bears a negative charge that interferes with phagocytosis. It also blocks complement deposition on the cell surface. Mutations during the course of infection alter the structure of M proteins, rendering some antibodies ineffective. Strains that persist in carriers frequently exhibit altered M proteins.
Leukocidins, including streptolysin S and streptolysin O, are proteins secreted by the streptococci to kill phagocytes (and probably to release nutrients for their growth)
Defense against host immune responses
Antigenic disguise and tolerance provided by hyaluronic acid capsule
Antigenic variation. Antibody against M protein (antigen) is the only effective protective antibody, but there are more than 50 different M types, and subsequent infections may occur with a different M serotype.
Production of toxins and other systemic effects
Toxic shock: Exotoxin is superantigen that binds directly to MHC II (without being processed) and binds abnormally to the T cell receptor of many (up to 20% of) T cells. Exaggerated production of cytokines causes the signs of shock: fever, rash, low blood pressure. aberrant interaction between toxin, macrophage, and T cells.
Induction of circulating, cross-reactive antibodies
Some of the antibodies produced during infection by certain strains of streptococci cross-react with certain host tissues. These antibodies can indirectly damage host tissues, even after the organisms have been cleared, and cause autoimmune complications.
Table 2. Summary of diseases caused by Streptococcus pyogenesSuppurative conditions (active infections associated with pus) occur in the throat, skin, and systemically.
Throat
Streptococcal pharyngitis is acquired by inhaling aerosols emitted by infected individuals. The symptoms reflect the inflammatory events at the site of infection. A few (1-3%) people develop rheumatic fever weeks after the infection has cleared.
Skin
Impetigo involves the infection of epidermal layers of skin. Pre-pubertal children are the most susceptible. Cellulitis occurs when the infection spreads subcutaneous tissues. Erysipelas is the infection of the dermis. About 5% of patients will develop more disseminated disease. Necrotizing fasciitis involves infection of the fascia and may proceed rapidly to underlying muscle.
Systemic
Scarlet fever is caused by production of erythrogenic toxin by a few strains of the organism.
Toxic shock is caused by a few strains that produce a toxic shock-like toxin.
Non-suppurative Sequelae
Some of the antibodies produced during the above infections cross-react with certain host tissues. These can indirectly damage host tissues, even after the organisms have beencleared, and cause non suppurative complications.
Rheumatic fever. M protein cross reacts with sarcolemma. Antibodies cross-react with heart tissue, fix complement, and cause damage.

Critical point dried whole group A streptococci (Streptococcus pyogenes) viewed directly by transmission electron microscopy (TEM 6,500X). Chains of streptococci are clearly evident. To remove cell surface proteins, cells were treated with trypsin prior to preparation and mounting. Strain: D471; M-type 6. Electron micrograph of Streptococcus pyogenes by Maria Fazio and Vincent A. Fischetti, Ph.D. with permission. The Laboratory of Bacterial Pathogenesis and Immunology, Rockefeller University.

Dividing streptococci (12,000X). Electron micrograph of Streptococcus pyogenes by Maria Fazio and Vincent A. Fischetti, Ph.D. with permission.The Laboratory of Bacterial Pathogenesis and Immunology, Rockefeller University.

Electron micrograph of an ultra-thin section of a chain of group A streptococci (20,000X). The cell surface fibrils, consisting primarily of M protein, are clearly evident. The bacterial cell wall, to which the fibrils are attached, is also clearly seen as the light staining region between the fibrils and the dark staining cell interior. Incipient cell division is also indicated by the nascent septum formation (seen as an indentation of the cell wall) near the cell equator. The streptococcal cell diameter is equal to approximately one micron. Electron micrograph of Streptococcus pyogenes by Maria Fazio and Vincent A. Fischetti, Ph.D. with permission.The Laboratory of Bacterial Pathogenesis and Immunology, Rockefeller University.

Negative staining of group A streptococci viewed by TEM 28,000X. The "halo" around the chain of cells (approximately equal in thickness to the cell diameter) is the remnants of the capsule that may be found surrounding the exterior of certain strains of group A streptococci. The septa between pairs of dividing cells may also be seen. Electron micrograph of Streptococcus pyogenes by Maria Fazio and Vincent A. Fischetti, Ph.D. with permission. The Laboratory of Bacterial Pathogenesis and Immunology, Rockefeller University.

High magnification electron micrograph of an ultra-thin section of a group A streptococcus sibling pair (70,000 X). At this magnification, especially in the cell on the left, the cell wall and cell surface fibrils, consisting primarily of M protein, are well defined. Interdigitaion of these fibrils between neighboring cells of different chains is also in plain view. Strain: C126/21/1; M-type 43. Electron micrograph of Streptococcus pyogenes by Maria Fazio and Vincent A. Fischetti, Ph.D. with permission.The Laboratory of Bacterial Pathogenesis and Immunology, Rockefeller University.

Figure 1. Streptococcus pyogenes. Left. Gram stain of Streptococcus pyogenes in a clinical specimen. Right. Colonies of Streptococcus pyogenes on blood agar exhibiting beta (clear) hemolysis.
Streptococcus pyogenes is one of the most frequent pathogens of humans. It is estimated that between 5-15% of normal individuals harbor the bacterium, usually in the respiratory tract, without signs of disease. As normal flora, S. pyogenes can infect when defenses are compromised or when the organisms are able to penetrate the constitutive defenses. When the bacteria are introduced or transmitted to vulnerable tissues, a variety of types of suppurative infectionscan occur.
In the last century, infections by S. pyogenes claimed many lives especially since the organism was the most important cause of puerperal fever (sepsis after childbirth). Scarlet fever was formerly a severe complication of streptococcal infection, but now, because of antibiotic therapy, it is little more than streptococcal pharyngitis accompanied by rash. Similarly, erysipelas (a form of cellulitis accompanied by fever and systemic toxicity) is less common today. However, there has been a recent increase in variety, severity andsequelae of Streptococcus pyogenes infections, and a resurgence of severe invasive infections, prompting descriptions of "flesh eating bacteria" in the news media. A complete explanation for the decline and resurgence is not known. Today, the pathogen is of major concern because of the occasional cases of rapidly progressive disease and because of the small risk of serious sequelae in untreated infections. These diseases remain a major worldwide health concern, and effort is being directed toward clarifying the risk and mechanisms of these sequelae and identifying rheumatogenic and nephritogenic strains of streptococci.
Acute Streptococcus pyogenes infections may present as pharyngitis (strep throat), scarlet fever (rash), impetigo (infection of the superficial layers of the skin) or cellulitis (infection of the deep layers of the skin). Invasive, toxigenic infections can result in necrotizing fasciitis, myositis and streptococcal toxic shock syndrome. Patients may also develop immune-mediated post-streptococcal sequelae, such as acute rheumatic fever and acuteglomerulonephritis, following acute infections caused by Streptococcus pyogenes.
Streptococcus pyogenes produces a wide array of virulence factors and a very large number of diseases. Virulence factors of Group A streptococci include: (1)M protein, fibronectin-binding protein (Protein F) and lipoteichoic acid for adherence; (2) hyaluronic acid capsule as an immunological disguise and to inhibit phagocytosis; M-protein to inhibit phagocytosis (3) invasins such asstreptokinase, streptodornase (DNase B), hyaluronidase, andstreptolysins; (4) exotoxins, such as pyrogenic (erythrogenic) toxin which causes the rash of scarlet fever and systemic toxic shock syndrome.
Classification of Streptococci
Hemolysis on blood agar
The type of hemolytic reaction displayed on blood agar has long been used to classify the streptococci. Beta -hemolysis is associated with complete lysis of red cells surrounding the colony, whereas alpha-hemolysis is a partial or "green" hemolysis associated with reduction of red cell hemoglobin. Nonhemolytic colonies have been termed gamma-hemolytic. Hemolysis is affected by the species and age of red cells, as well as by other properties of the base medium. Group A streptococci are nearly always beta-hemolytic; related Group B can manifest alpha, beta or gamma hemolysis. Most strains ofS. pneumoniae are alpha-hemolytic but can cause ß-hemolysis during anaerobic incubation. Most of the oral streptococci and enterococci are non hemolytic. The property of hemolysis is not very reliable for the absolute identification of streptococci, but it is widely used in rapid screens for identification of S. pyogenes and S. pneumoniae.
Antigenic types
The cell surface structure of Group A streptococci is among the most studied of any bacteria (Figure 2). The cell wall is composed of repeating units of N-acetylglucosamine and N-acetylmuramic acid, the standard peptidoglycan. Historically, the definitive identification of streptococci has rested on the serologic reactivity of "cell wall" polysaccharide antigens as originally described by Rebecca Lancefield. Eighteen group-specific antigens (Lancefield groups) were established. The Group A polysaccharide is a polymer of N-acetylglucosamine and rhamnose. Some group antigens are shared by more than one species. This polysaccharide is also called the C substance or group carbohydrate antigen.
Pathogenesis
Streptococcus pyogenes owes its major success as a pathogen to its ability to colonize and rapidly multiply and spread in its host while evading phagocytosis and confusing the immune system.
Acute diseases associated with Streptococcus pyogenes occur chiefly in therespiratory tract, bloodstream, or the skin. Streptococcal disease is most often a respiratory infection (pharyngitis or tonsillitis) or a skin infection (pyoderma). Some strains of streptococci show a predilection for the respiratory tract; others, for the skin. Generally, streptococcal isolates from the pharynx and respiratory tract do not cause skin infections. Figure 3 describes the pathogenesis of S. pyogenes infections.
S. pyogenes is the leading cause of uncomplicated bacterial pharyngitis andtonsillitis commonly referred to a strep throat. Other respiratory infections include sinusitis, otitis, and pneumonia.
Infections of the skin can be superficial (impetigo) or deep (cellulitis). Invasive streptococci cause joint or bone infections, destructive wound infections (necrotizing fasciitis) and myositis, meningitis andendocarditis. Two post streptococcal sequelae, rheumatic fever andglomerulonephritis, may follow streptococcal disease, and occur in 1-3% of untreated infections. These conditions and their pathology are not attributable to dissemination of bacteria, but to aberrent immunological reactions to Group A streptococcal antigens. Scarlet fever and streptococcal toxic shock syndromeare systemic responses to circulating bacterial toxins.
Infections of the skin can be superficial (impetigo) or deep (cellulitis). Invasive streptococci cause joint or bone infections, destructive wound infections (necrotizing fasciitis) and myositis, meningitis andendocarditis. Two post streptococcal sequelae, rheumatic fever andglomerulonephritis, may follow streptococcal disease, and occur in 1-3% of untreated infections. These conditions and their pathology are not attributable to dissemination of bacteria, but to aberrent immunological reactions to Group A streptococcal antigens. Scarlet fever and streptococcal toxic shock syndromeare systemic responses to circulating bacterial toxins.
The cell surface of Streptococcus pyogenes accounts for many of the bacterium's determinants of virulence, especially those concerned with colonization and evasion of phagocytosis and the host immune responses. The surface of Streptococcus pyogenes is incredibly complex and chemically-diverse. Antigenic components include capsular polysaccharide (C-substance), cell wall peptidoglycan and lipoteichoic acid (LTA), and a variety of surface proteins, including M protein, fimbrial proteins, fibronectin-binding proteins, (e.g. Protein F) and cell-bound streptokinase.
The cytoplasmic membrane of S. pyogenes contains some antigens similar to those of human cardiac, skeletal, and smooth muscle, heart valve fibroblasts, and neuronal tissues, resulting in molecular mimicry and a tolerant or suppressed immune response by the host.
The cell envelope of a Group A streptococcus is illustrated in Figure 2. The complexity of the surface can be seen in several of the electron micrographs of the bacterium that accompany this article.

Figure 2. Cell surface structure of Streptococcus pyogenes and secreted products involved in virulence.
In Group A streptococci, the R and T proteins are used as epidemiologic markers and have no known role in virulence. The group carbohydrate antigen (composed of N-acetylglucosamine and rhamnose) has been thought to have no role in virulence, but emerging strains with increased invasive capacity produce a very mucoid colony, suggesting a role of the capsule in virulence.
The M proteins are clearly virulence factors associated with both colonization and resistance to phagocytosis. More than 50 types of S. pyogenes M proteins have been identified on the basis of antigenic specificity, and it is the M protein that is the major cause of antigenic shift and antigenic drift in the Group A streptococci. The M protein (found in fimbriae) also binds fibrinogen from serum and blocks the binding of complement to the underlying peptidoglycan. This allows survival of the organism by inhibiting phagocytosis.
The streptococcal M protein, as well as peptidoglycan, N-acetylglucosamine, and group-specific carbohydrate, contain antigenic epitopes that mimic those of mammalian muscle and connective tissue. As mentioned above, the cell surface of recently emerging strains of streptococci is distinctly mucoid (indicating that they are highly encapsulated). These strains are also rich in surface M protein. The M proteins of certain M-types are considered rheumatogenic since they contain antigenic epitopes related to heart muscle, and they therefore may lead to autoimmune rheumatic carditis (rheumatic fever) following an acute infection.
The Hyaluronic Acid Capsule
The capsule of S. pyogenes is non antigenic since it is composed of hyaluronic acid, which is chemically similar to that of host connective tissue. This allows the bacterium to hide its own antigens and to go unrecognized as antigenic by its host. The Hyaluronic acid capsule also prevents opsonized phagocytosis by neutrophils or mancrophages.
Adhesins
Colonization of tissues by S. pyogenes is thought to result from a failure in the constitutive defenses (normal flora and other nonspecific defense mechanisms) which allows establishment of the bacterium at a portal of entry (often the upper respiratory tract or the skin) where the organism multiplies and causes an inflammatory purulent lesion.
It is now realized that S. pyogenes (like many other bacterial pathogens) produces multiple adhesins with varied specificities. There is evidence thatStreptococcus pyogenes utilizes lipoteichoic acids (LTA), M protein, and multiple fibronectin-binding proteins in its repertoire of adhesins. LTA is anchored to proteins on the bacterial surface, including the M protein. Both the M proteins and lipoteichoic acid are supported externally to the cell wall on fimbriae and appear to mediate bacterial adherence to host epithelial cells. The fibronectin-binding protein, Protein F, has also been shown to mediate streptococcal adherence to the amino terminus of fibronectin on mucosal surfaces.
Identification of Streptococcuspyogenes adhesins has long been a subject of conflict and debate. Most of the debate was between proponents of the LTA model and those of the M protein model. In 1972, Gibbons and his colleagues proposed that attachment of streptococci to the oral mucosa of mice is dependent on M protein. However, Olfek and Beachey argued that lipoteichoic acid (LTA), rather than M protein, was responsible for streptococcal adherence to buccal epithelial cells. In 1996, Hasty and Courtney proposed a two-step model of attachment that involved both M protein and teichoic acids. They suggested that LTA loosely tethers streptococci to epithelial cells, and then M protein and/or other fibronectin (Fn)-binding proteins secure a firmer, irreversible association. The first streptococcal fibronectin-binding protein (Sfb) was demonstrated in 1992. Shortly thereafter, protein F was discovered. Most recently (1998), the M1 and M3 proteins were shown to bind fibronectin.
Extracellular products: invasins and exotoxins
Colonization of the upper respiratory tract and acute pharyngitis may spread to other portions of the upper or lower respiratory tracts resulting in infections of the middle ear (otitis media), sinuses (sinusitis), or lungs (pneumonia). In addition, meningitis can occur by direct extension of infection from the middle ear or sinuses to the meninges or by way of bloodstream invasion from the pulmonary focus. Bacteremia can also result in infection of bones (osteomyelitis) or joints (arthritis). During these aspects of acute disease the streptococci bring into play a variety of secretory proteins that mediate their invasion.
For the most part, streptococcal invasins and protein toxins interact with mammalian blood and tissue components in ways that kill host cells and provoke a damaging inflammatory response. The soluble extracellular growth products and toxins of Streptococcus pyogenes (see Figure 2, above), have been studied intensely. Streptolysin S is an oxygen-stable leukocidin; Streptolysin O is an oxygen-labile leukocidin. NADase is also leukotoxic. Hyaluronidase (the original "spreading factor") can digest host connective tissue hyaluronic acid, as well as the organism's own capsule. Streptokinases participate in fibrin lysis.Streptodornases A-D possess deoxyribonuclease activity; Streptodornases B and D possess ribonuclease activity as well. Protease activity similar to that inStaphylococcus aureus has been shown in strains causing soft tissue necrosis or toxic shock syndrome. This large repertoire of products is important in the pathogenesis of S. pyogenes infections. Even so, antibodies to these products are relatively insignificant in protection of the host.
The streptococcal invasins act in a variety of ways summarized in Table 1 at the end of this article. Streptococcal invasins lyse eukaryotic cells, including red blood cells and phagocytes; they lyse other host macromolecules, including enzymes and informational molecules; they allow the bacteria to spread among tissues by dissolving host fibrin and intercellular ground substances.
Pyrogenic Exotoxins
Three streptococcal pyrogenic exotoxins (SPE), formerly known asErythrogenic toxin, are recognized: types A, B, C. These toxins act assuperantigens by a mechanism similar to those described for staphylococci. As antigens, they do not requiring processing by antigen presenting cells. Rather, they stimulate T cells by binding class II MHC molecules directly and nonspecifically. With superantigens about 20% of T cells may be stimulated (vs 1/10,000 T cells stimulated by conventional antigens) resulting in massive detrimental cytokine release. SPE A and SPE C are encoded by lysogenic phages; the gene for SPE B is located on the bacterial chromosome.
The erythrogenic toxin is so-named for its association with scarlet fever which occurs when the toxin is disseminated in the blood. Re-emergence in the late 1980's of exotoxin-producing strains of S. pyogenes has been associated with atoxic shock-like syndrome similar in pathogenesis and manifestation to staphylococcal toxic shock syndrome, and with other forms of invasive disease associated with severe tissue destruction. The latter condition is termednecrotizing fasciitis. Outbreaks of sepsis, toxic shock and necrotizing fasciitis have been reported at increasing frequency. The destructive nature of wound infections prompted the popular press to refer to S. pyogenes as "flesh-eating bacteria" and "skin-eating streptococci". The increase in invasive streptococcal disease was associated with emergence of a highly virulent serotype M1 which is disseminated world-wide. The M1 strain produces the erythrogenic toxin (Spe A), thought to be responsible for toxic shock, and the enzyme cysteine protease which is involved in tissue destruction. Because clusters of toxic shock were also associated with other serotypes, particularly M3 strains, it is believed that unidentified host factors may also have played an important role in the resurgence of these dangerous infections.

FIGURE 3. Pathogenesis of Streptococcus pyogenes infections. Adapted from Baron's Medical Microbiology Chapter 13, Streptococcus by Maria Jevitz Patterson.
Post streptococcal sequelae
Infection with Streptococcus pyogenes can give rise to serious nonsuppurative sequelae: acute rheumatic fever and acute glomerulonephritis. These pathological events begin 1-3 weeks after an acute streptococcal illness, a latent period consistent with an immune-mediated etiology. Whether all S. pyogenesstrains are rheumatogenic is controversial; however, clearly not all strains are nephritogenic.
Acute rheumatic fever is a sequel only of pharyngeal infections, but acuteglomerulonephritis can follow infections of the pharynx or the skin. Although there is no adequate explanation for the precise pathogenesis of acute rheumatic fever, an abnormal or enhanced immune response seems essential. Also, persistence of the organism on pharyngeal tissues (i.e., the tonsils) is associated with an increased likelihood of rheumatic fever. Acute rheumatic fever can result in permanent damage to the heart valves. Less than 1% of sporadic streptococcal pharyngitis infections result in acute rheumatic fever; however, recurrences are common, and life-long antibiotic prophylaxis is recommended following a single case.Infection with Streptococcus pyogenes can give rise to serious nonsuppurative sequelae: acute rheumatic fever and acute glomerulonephritis. These pathological events begin 1-3 weeks after an acute streptococcal illness, a latent period consistent with an immune-mediated etiology. Whether all S. pyogenesstrains are rheumatogenic is controversial; however, clearly not all strains are nephritogenic.
The occurrence of cross-reactive antigens in S. pyogenes and heart tissues possibly explains the autoimmune responses that develop following some infections. The antibody mediated immune (AMI) response (i.e., level of serum antibody) is higher in patients with rheumatic fever than in patients with uncomplicated pharyngitis. In addition, cell-mediated immunity (CMI) seems to play a role in the pathology of acute rheumatic fever.
Acute glomerulonephritis results from deposition of antigen-antibody-complement complexes on the basement membrane of kidney glomeruli. The antigen may be streptococcal in origin or it may be a host tissue species with antigenic determinants similar to those of streptococcal antigen (cross-reactive epitopes for endocardium, sarcolemma, vascular smooth muscle). The incidence of acute glomerulonephritis in the United States is variable, perhaps due to cycling of nephritogenic strains, but it appears to be decreasing. Recurrences are uncommon, and prophylaxis following an initial attack is unnecessary.
Host defenses
S. pyogenes is usually an exogenous secondary invader, following viral disease or disturbances in the normal bacterial flora. In the normal human the skin is an effective barrier against invasive streptococci, and nonspecific defense mechanisms prevent the bacteria from penetrating beyond the superficial epithelium of the upper respiratory tract. These mechanisms include mucociliary movement, coughing, sneezing and epiglottal reflexes.
The host phagocytic system is a second line of defense against streptococcal invasion. Organisms can be opsonized by activation of the classical or alternate complement pathway and by anti-streptococcal antibodies in the serum. S. pyogenes is rapidly killed following phagocytosis enhanced by specific antibody. The bacteria do not produce catalase or significant amounts of superoxide dismutase to inactivate the oxygen metabolites (hydrogen peroxide, superoxide) produced by the oxygen-dependent mechanisms of the phagocyte. Therefore, they are quickly killed after engulfment by phagocytes. The streptococcal defense must be one to stay out of phagocytes.S. pyogenes is usually an exogenous secondary invader, following viral disease or disturbances in the normal bacterial flora. In the normal human the skin is an effective barrier against invasive streptococci, and nonspecific defense mechanisms prevent the bacteria from penetrating beyond the superficial epithelium of the upper respiratory tract. These mechanisms include mucociliary movement, coughing, sneezing and epiglottal reflexes.
In immune individuals, IgG antibodies reactive with M protein promote phagocytosis which results in killing of the organism. This is the major mechanism by which AMI is able to terminate Group A streptococcal infections.M protein vaccines are a major candidate for use against rheumatic fever, but certain M protein types cross-react antigenically with the heart and themselves may be responsible for rheumatic carditis. This risk of autoimmunity has prevented the use of Group A streptococcal vaccines. However, since the cross-reactive epitopes of the M-protein are now known, it appears that limited anti-streptococcal vaccines are on the horizon.

FIGURE 4. Phagocytosis of Streptococcus pyogenes by a macrophage.CELLS alive!
The hyaluronic acid capsule allows the organism to evade opsonization. The capsule is also an antigenic disguise that hides bacterial antigens and is non antigenic to the host. Actually, the hyaluronic acid outer surface of S. pyogenesis weakly antigenic, but it does not result in stimulation of protective immunity. The only protective immunity that results from infection by Group A streptococcus comes from the development of type-specific antibody to the M protein of the fimbriae, which protrude from the cell wall through the capsular structure. This antibody, which follows respiratory and skin infections, is persistent. Presumably, protective levels of specific IgA is produced in the respiratory secretions while protective levels of IgG are formed in the serum. Sometimes, intervention of an infection with effective antibiotic treatment precludes the development of this persistent antibody. This accounts, in part, for recurring infections in an individual by the same streptococcal strain. Antibody to the erythrogenic toxin involved in scarlet fever is also long lasting.
Treatment and prevention
Penicillin is still uniformly effective in treatment of Group A streptococcal disease. It is important to identify and treat Group A streptococcal infections in order to prevent sequelae. No effective vaccine has been produced, but specific M-protein vaccines are being tested.
Penicillin is still uniformly effective in treatment of Group A streptococcal disease. It is important to identify and treat Group A streptococcal infections in order to prevent sequelae. No effective vaccine has been produced, but specific M-protein vaccines are being tested.
Table 1. Summary of virulence determinants of Streptococcus pyogenes
M protein
Lipoteichoic acid (LTA)
Protein F and Sfb (fibronectin-binding proteins)
Enhancement of spread in tissues
Hyaluronidase hydrolyses hyaluronic acid, part of the ground substance in host tissues.
Proteases
Streptokinase lyses fibrin
Evasion of phagocytosis
Capsule: hyaluronic acid is produced.
C5a peptidase: C5a enhances chemotaxis of phagocytes .
M protein is a fibrillar surface protein. Its distal end bears a negative charge that interferes with phagocytosis. It also blocks complement deposition on the cell surface. Mutations during the course of infection alter the structure of M proteins, rendering some antibodies ineffective. Strains that persist in carriers frequently exhibit altered M proteins.
Leukocidins, including streptolysin S and streptolysin O, are proteins secreted by the streptococci to kill phagocytes (and probably to release nutrients for their growth)
Defense against host immune responses
Antigenic disguise and tolerance provided by hyaluronic acid capsule
Antigenic variation. Antibody against M protein (antigen) is the only effective protective antibody, but there are more than 50 different M types, and subsequent infections may occur with a different M serotype.
Production of toxins and other systemic effects
Toxic shock: Exotoxin is superantigen that binds directly to MHC II (without being processed) and binds abnormally to the T cell receptor of many (up to 20% of) T cells. Exaggerated production of cytokines causes the signs of shock: fever, rash, low blood pressure. aberrant interaction between toxin, macrophage, and T cells.
Induction of circulating, cross-reactive antibodies
Some of the antibodies produced during infection by certain strains of streptococci cross-react with certain host tissues. These antibodies can indirectly damage host tissues, even after the organisms have been cleared, and cause autoimmune complications.
Table 2. Summary of diseases caused by Streptococcus pyogenes
Throat
Streptococcal pharyngitis is acquired by inhaling aerosols emitted by infected individuals. The symptoms reflect the inflammatory events at the site of infection. A few (1-3%) people develop rheumatic fever weeks after the infection has cleared.
Skin
Impetigo involves the infection of epidermal layers of skin. Pre-pubertal children are the most susceptible. Cellulitis occurs when the infection spreads subcutaneous tissues. Erysipelas is the infection of the dermis. About 5% of patients will develop more disseminated disease. Necrotizing fasciitis involves infection of the fascia and may proceed rapidly to underlying muscle.
Systemic
Scarlet fever is caused by production of erythrogenic toxin by a few strains of the organism.
Toxic shock is caused by a few strains that produce a toxic shock-like toxin.
Non-suppurative Sequelae
Some of the antibodies produced during the above infections cross-react with certain host tissues. These can indirectly damage host tissues, even after the organisms have beencleared, and cause non suppurative complications.
Rheumatic fever. M protein cross reacts with sarcolemma. Antibodies cross-react with heart tissue, fix complement, and cause damage.
Glomerulonephritis. Antigen-antibody complexes may be deposited in kidney, fix complement, and damage glomeruli. Only a few M-types are nephritogenic.
Gallery of electron micrographs of Streptococcus pyogenes from The Laboratory of Pathogenesis and Immunology at Rockefeller University, the home of research on Streptococcus pyogenes

Critical point dried whole group A streptococci (Streptococcus pyogenes) viewed directly by transmission electron microscopy (TEM 6,500X). Chains of streptococci are clearly evident. To remove cell surface proteins, cells were treated with trypsin prior to preparation and mounting. Strain: D471; M-type 6. Electron micrograph of Streptococcus pyogenes by Maria Fazio and Vincent A. Fischetti, Ph.D. with permission. The Laboratory of Bacterial Pathogenesis and Immunology, Rockefeller University.

Dividing streptococci (12,000X). Electron micrograph of Streptococcus pyogenes by Maria Fazio and Vincent A. Fischetti, Ph.D. with permission.The Laboratory of Bacterial Pathogenesis and Immunology, Rockefeller University.

Electron micrograph of an ultra-thin section of a chain of group A streptococci (20,000X). The cell surface fibrils, consisting primarily of M protein, are clearly evident. The bacterial cell wall, to which the fibrils are attached, is also clearly seen as the light staining region between the fibrils and the dark staining cell interior. Incipient cell division is also indicated by the nascent septum formation (seen as an indentation of the cell wall) near the cell equator. The streptococcal cell diameter is equal to approximately one micron. Electron micrograph of Streptococcus pyogenes by Maria Fazio and Vincent A. Fischetti, Ph.D. with permission.The Laboratory of Bacterial Pathogenesis and Immunology, Rockefeller University.

Negative staining of group A streptococci viewed by TEM 28,000X. The "halo" around the chain of cells (approximately equal in thickness to the cell diameter) is the remnants of the capsule that may be found surrounding the exterior of certain strains of group A streptococci. The septa between pairs of dividing cells may also be seen. Electron micrograph of Streptococcus pyogenes by Maria Fazio and Vincent A. Fischetti, Ph.D. with permission. The Laboratory of Bacterial Pathogenesis and Immunology, Rockefeller University.

High magnification electron micrograph of an ultra-thin section of a group A streptococcus sibling pair (70,000 X). At this magnification, especially in the cell on the left, the cell wall and cell surface fibrils, consisting primarily of M protein, are well defined. Interdigitaion of these fibrils between neighboring cells of different chains is also in plain view. Strain: C126/21/1; M-type 43. Electron micrograph of Streptococcus pyogenes by Maria Fazio and Vincent A. Fischetti, Ph.D. with permission.The Laboratory of Bacterial Pathogenesis and Immunology, Rockefeller University.
END OF CHAPTER
My little princess is so beautiful) I underwent using Dr Itua Herbal Medicine. I had a miscarriage 7 years ago. I still can’t hold back my tears when I remember that horrible period of my life. After my loss I couldn’t get back to life for a long time. I’m glad I have my husband. He gave me support I needed the most. Together we can do everything! We wanted to have kids for a really long time. We’ve gone through a lot, but if you want something badly, you’ll get it! I had to search online on how i can use herbal remedy due to my infertility then i came across Dr Itua how he cure all kind diseases and helped a lady from Kansas City to get pregnant so i contacted him on email,He gave me some guild lines to follow he also send me his herbal medicine via courier service which he instruct me on how to drink it for two weeks really i did and after 7 days of having intercourse with my husband few days later i noticed my period didn't come then i decided to go for check up i was pregnant with a baby,Dr Itua is a genuine miracle man..I've got pregnant from first attempt. We were over the moon! Our girls were born in May 2015. We've just celebrated their first birthday. Finally joy and peace came to our family.Here his Email/Whatsapp Number...+2348149277967/ drituaherbalcenter@gmail.com He cure the following...infertility Liver/kidney Inflammatory,Diabetis,Herpes Virus, Lupus, HPV, Cancer, Hiv/Aids, Hepatitis, Als, MS, Menstrual Cramp, Fribroid.
ReplyDelete