Borrelia burgdorferi and Lyme Disease
Borrelia burgdorferi, the spirochete that causes Lyme Disease. FA stain (CDC)
Introduction
Lyme disease was first recognized in the United States in 1975 by Dr. Allen Steere, following a mysterious outbreak of juvenile rheumatoid arthritis near the community of Lyme, Connecticut. The rural location of the Lyme outbreak and the onset of illness during summer and early fall suggested that the transmission of the disease was by an arthropod vector.
In 1982, the etiologic agent of Lyme disease was discovered by Willy Burgdorfer, who isolated spirochetes belonging to the genus Borrelia from the mid-guts of Ixodes ticks. He showed that these spirochetes reacted with immune serum from patients that had been diagnosed with Lyme disease. Subsequently, the etiologic agent was given the name Borrelia burgdorferi. Since then, reports of Lyme disease have increased dramatically to the point that the disease has become an important public health problem in some areas of the United States. Today, Lyme disease is the most prevalent tick-borne illness in the United States.

Incidence of Lyme Disease in the United States, 1991-2006. Lyme disease is the most prevalent tick-borne illness in the United States. In 2006, there were 19,931 new cases reported. Between 1996 and 2001 the average number was about 17,000 new cases per year but increased to near or above 20,000 new cases per year in 2002, probably due to increased surveillance and reporting. CDC.
Biology of Spirochetes
Borrelia burgdorferi, like the human pathogen Treponema pallidum, is aspirochete. Spirochetes are a group of phylogenetically-distinct bacteria that have a unique mode of motility by means of axial filaments (endoflagella). Spirochetes are widespread in viscous environments and they are found in the intestinal tracts of animals and the oral cavity of humans. The spirochetes have a unique cell surface which accompanies their unique type of motility. The endoflagella are contained within the periplasmic space between a semi rigid peptidoglycan helix and a multi-layer, flexible outer membrane sheath. When the filaments rotate within this space, the spirochetes move in cork-screw fashion. This type of movement is thought to be an adaptation to viscous environments, such as aquatic sediments, biofilms, mucosal tissues and the intestinal tracts of animals. For pathogens, this allows the spirochetes to hide their flagella, which are normally antigenic, from the host immune defenses.

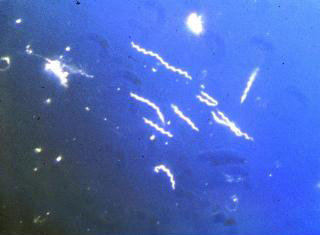
Spirochetes are usually much longer than they are wide, and often their width is below the resolving power of the light microscope. For example, Borrelia may have a length of 20-30um but a width of only 0.2-0.3um. Hence, most spirochetes cannot be viewed using conventional light microscopy. Dark-field microscopy must be used to view spirochetes. Dark field microscopy utilizes a special condenser which directs light toward an object at a angle, rather than from the bottom. As a result, particles or cells are seen as light objects against a dark background.
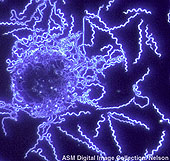
B. burgdorferi dark field illumination. American society for Microbiology.
The spirochetes are not classified as either Gram-positive or Gram-negative. When Borrelia burgdorferi is Gram-stained, the cells stain a weak Gram-negative by default, as safranin is the last dye used. Borrelia, like most spirochetes, does have an outer membrane that contains an LPS-like substance, an inner membrane, and a periplasmic space which contains a layer of peptidoglycan. Therefore, it has a Gram-negative bacterial type cell wall, despite its staining characteristics.
Cultivation
Unlike Treponema pallidum, Borrelia burgdorferi can be cultivated in vitro. However, the bacterium is fastidious and requires a very complex growth medium. The medium used to grow Borrelia burgdorferi is called Barbour-Stoenner-Kelly (BSK) medium. It contains over thirteen ingredients in a rabbit serum base. Borrelia burgdorferi has an optimal temperature for growth of 32oC, in a microaerobic environment. Even under optimal conditions, the generation time is slow, about 12-24 hours.
Borreliae from ticks and from the blood, skin, and cerebrospinal fluid of Lyme disease patients have been successfully cultivated in BSK medium. BSK solidified with 1.3% agarose allows the production of colonies from single organisms.
Strains of Borrelia
The borreliae causing Lyme disease are divided into several "genospecies", three of which have been firmly established and are well accepted:
I. Borrelia burgdorferi sensu stricto
II. Borrelia garinii
III. Borrelia afzelii
The term used to collectively describe all three genospecies is Borrelia burgdorferi sensu lato. The differences in genospecies are revealed by restriction fragment length polymorphism, (RFLP), multi-locus enzyme electrophoresis (MLEE) and ssRNA sequences. All U.S. isolates fall into genospecies I. Examples of all three genospecies have been found in Europe and Asia, although II and III predominate there.
Outer Surface Proteins
The outer membrane of Borrelia burgdorferi is composed of various unique outer surface proteins (Osp) that have been characterized (Osp A through OspF). They are presumed to play a role in virulence. Osp A and Osp B are by far the most abundant outer surface proteins. The genes encoding these proteins are transcribed from a common promoter and are located on a 49 kb linear plasmid. The chromosome of Borrelia burgdorferi is also linear and is almost 1100 kb in size.
Incidence and Distribution of Lyme Disease in the United States
Lyme disease has a wide distribution in northern temperate regions of the world. In the United States, the highest incidence occurs in the Northeast, from Massachusetts to Maryland and the North-central states, especially Wisconsin and Minnesota.
In 2002, more than 23,000 cases of Lyme disease were reported in the U.S., the highest number ever reported. This increase could be caused by an increase in human contact with infected ticks and enhanced reporting of cases.
Ten states have consistently reported an incidence of Lyme disease higher than the national average: Connecticut, Delaware, Maryland, Massachusetts, Minnesota, New Jersey, New York, Pennsylvania, Rhode Island and Wisconsin. During 2003-2005, 64,382 Lyme disease cases were reported to CDC, of which 59,770 cases (93%) were reported from these 10 states. Similar data was reported in 2006 (see map below).

Lyme disease cases by state, 2006. CDC.

Borrelia burgdorferi, the spirochete that causes Lyme Disease. FA stain (CDC)
Introduction
Lyme disease was first recognized in the United States in 1975 by Dr. Allen Steere, following a mysterious outbreak of juvenile rheumatoid arthritis near the community of Lyme, Connecticut. The rural location of the Lyme outbreak and the onset of illness during summer and early fall suggested that the transmission of the disease was by an arthropod vector.
In 1982, the etiologic agent of Lyme disease was discovered by Willy Burgdorfer, who isolated spirochetes belonging to the genus Borrelia from the mid-guts of Ixodes ticks. He showed that these spirochetes reacted with immune serum from patients that had been diagnosed with Lyme disease. Subsequently, the etiologic agent was given the name Borrelia burgdorferi. Since then, reports of Lyme disease have increased dramatically to the point that the disease has become an important public health problem in some areas of the United States. Today, Lyme disease is the most prevalent tick-borne illness in the United States.

Incidence of Lyme Disease in the United States, 1991-2006. Lyme disease is the most prevalent tick-borne illness in the United States. In 2006, there were 19,931 new cases reported. Between 1996 and 2001 the average number was about 17,000 new cases per year but increased to near or above 20,000 new cases per year in 2002, probably due to increased surveillance and reporting. CDC.
Biology of Spirochetes
Borrelia burgdorferi, like the human pathogen Treponema pallidum, is aspirochete. Spirochetes are a group of phylogenetically-distinct bacteria that have a unique mode of motility by means of axial filaments (endoflagella). Spirochetes are widespread in viscous environments and they are found in the intestinal tracts of animals and the oral cavity of humans. The spirochetes have a unique cell surface which accompanies their unique type of motility. The endoflagella are contained within the periplasmic space between a semi rigid peptidoglycan helix and a multi-layer, flexible outer membrane sheath. When the filaments rotate within this space, the spirochetes move in cork-screw fashion. This type of movement is thought to be an adaptation to viscous environments, such as aquatic sediments, biofilms, mucosal tissues and the intestinal tracts of animals. For pathogens, this allows the spirochetes to hide their flagella, which are normally antigenic, from the host immune defenses.

Spirochetes are usually much longer than they are wide, and often their width is below the resolving power of the light microscope. For example, Borrelia may have a length of 20-30um but a width of only 0.2-0.3um. Hence, most spirochetes cannot be viewed using conventional light microscopy. Dark-field microscopy must be used to view spirochetes. Dark field microscopy utilizes a special condenser which directs light toward an object at a angle, rather than from the bottom. As a result, particles or cells are seen as light objects against a dark background.

B. burgdorferi dark field illumination. American society for Microbiology.
The spirochetes are not classified as either Gram-positive or Gram-negative. When Borrelia burgdorferi is Gram-stained, the cells stain a weak Gram-negative by default, as safranin is the last dye used. Borrelia, like most spirochetes, does have an outer membrane that contains an LPS-like substance, an inner membrane, and a periplasmic space which contains a layer of peptidoglycan. Therefore, it has a Gram-negative bacterial type cell wall, despite its staining characteristics.
Cultivation
Unlike Treponema pallidum, Borrelia burgdorferi can be cultivated in vitro. However, the bacterium is fastidious and requires a very complex growth medium. The medium used to grow Borrelia burgdorferi is called Barbour-Stoenner-Kelly (BSK) medium. It contains over thirteen ingredients in a rabbit serum base. Borrelia burgdorferi has an optimal temperature for growth of 32oC, in a microaerobic environment. Even under optimal conditions, the generation time is slow, about 12-24 hours.
Borreliae from ticks and from the blood, skin, and cerebrospinal fluid of Lyme disease patients have been successfully cultivated in BSK medium. BSK solidified with 1.3% agarose allows the production of colonies from single organisms.
Strains of Borrelia
The borreliae causing Lyme disease are divided into several "genospecies", three of which have been firmly established and are well accepted:
I. Borrelia burgdorferi sensu stricto
II. Borrelia garinii
III. Borrelia afzelii
The term used to collectively describe all three genospecies is Borrelia burgdorferi sensu lato. The differences in genospecies are revealed by restriction fragment length polymorphism, (RFLP), multi-locus enzyme electrophoresis (MLEE) and ssRNA sequences. All U.S. isolates fall into genospecies I. Examples of all three genospecies have been found in Europe and Asia, although II and III predominate there.
Outer Surface Proteins
The outer membrane of Borrelia burgdorferi is composed of various unique outer surface proteins (Osp) that have been characterized (Osp A through OspF). They are presumed to play a role in virulence. Osp A and Osp B are by far the most abundant outer surface proteins. The genes encoding these proteins are transcribed from a common promoter and are located on a 49 kb linear plasmid. The chromosome of Borrelia burgdorferi is also linear and is almost 1100 kb in size.
Pathogenicity
Borrelia burgdorferi invades the blood and tissues of various infected mammals and birds. The natural reservoir for Borrelia burgdorferi is thought to be the white-footed mouse. Ticks transfer the spirochetes to the white-tailed deer, humans, and other warm-blooded animals after a blood meal on an infected animal. In humans, dogs, and many other animals, infection with Borrelia burgdorferi results in the pathology of Lyme disease.
Borrelia burgdorferi invades the blood and tissues of various infected mammals and birds. The natural reservoir for Borrelia burgdorferi is thought to be the white-footed mouse. Ticks transfer the spirochetes to the white-tailed deer, humans, and other warm-blooded animals after a blood meal on an infected animal. In humans, dogs, and many other animals, infection with Borrelia burgdorferi results in the pathology of Lyme disease.
Lyme disease has a wide distribution in northern temperate regions of the world. In the United States, the highest incidence occurs in the Northeast, from Massachusetts to Maryland and the North-central states, especially Wisconsin and Minnesota.
In 2002, more than 23,000 cases of Lyme disease were reported in the U.S., the highest number ever reported. This increase could be caused by an increase in human contact with infected ticks and enhanced reporting of cases.
Ten states have consistently reported an incidence of Lyme disease higher than the national average: Connecticut, Delaware, Maryland, Massachusetts, Minnesota, New Jersey, New York, Pennsylvania, Rhode Island and Wisconsin. During 2003-2005, 64,382 Lyme disease cases were reported to CDC, of which 59,770 cases (93%) were reported from these 10 states. Similar data was reported in 2006 (see map below).

Lyme disease cases by state, 2006. CDC.
Transmission of Lyme Disease
Lyme disease is spread by the bite of ticks of the genus Ixodes that are infected with Borrelia burgdorferi. Ixodes, commonly known as the deer tick (or bear tick), normally feeds on the white-footed mouse, the white-tailed deer, and certain other mammals. It is responsible for transmitting the spirochetes to humans in the northeastern and north-central United States. On the Pacific Coast, the bacteria are transmitted to humans by the western black-legged tick, and in the southeastern states by the related black-legged tick.

Distribution of Ixodes ticks that transmit Lyme disease in the U.S. CDC.
Ixodes ticks are much smaller than common dog and cattle ticks. In their larval and nymphal stages, they are no bigger than a pinhead. Adult ticks are slightly larger. The tick nymphs, which are most likely to feed on a person and are rarely noticed because of their small size (less than 2 mm), are usually involved in the transmission of the disease.

Ixodes ticks: larva, nymph, adult male, adult female. CDC.
Spirochete prevalence in adult Ixodes ticks is highly variable depending on geographic location. It was shown to be present in approximately 35% of ticks in the Baraboo Hills northwest of Madison, Wisconsin, while in regions of California, 2% prevalence has been reported, and in regions of New York, 50% has been reported.
For Lyme disease to exist in an area, at least three closely interrelated elements must be present in nature: the Lyme disease bacteria, Borrelia burgdorferi,ticks that can transmit them, and mammals (such as mice and deer) to provide food for the ticks in their various life stages.
The tick life cycle consists of three distinctive stages: larvae, nymphs, and adults. A blood meal is required for ticks to molt from the larvae stage to the nymph stage and from the nymph stage to the adult stage. The tick larvae and nymphs typically become infected with Borrelia burgdorferi when they feed on infected small animals, particularly the white-footed mouse. The bacteria remain in the tick as it changes from larva to nymph or from nymph to adult. Infected nymphs and adult ticks then bite and transmit the bacteria to other small rodents, other animals, and humans, all in the course of their normal feeding behavior. Adult ticks preferentially feed on the white-tailed deer, which thereby becomes an important reservoir in regions of infestation. The tick life cycle takes two years to complete (see diagram below).

Symptoms of Lyme disease
The symptoms of Lyme disease in humans occur in three stages.
Stage one (early infection). The early stage of Lyme disease is often characterized by a distinctive, expanding red rash that usually develops at the site of the tick bite. This rash, known as erythema migrans, is seen in 60-80% of infected individuals (it is important to remember that the converse is true: no rash is ever observed in 20-40% of the cases). Spirochetes can be isolated from the leading edge of the rash. Erythema migrans is a red circular patch that appears usually 3 days to 1 month following the bite of the tick. The patch then expands, often to a large size and develops a characteristic "bull's eye" appearance. However, not all rashes that occur at the site of a tick bite are due to Lyme disease. An allergic reaction to tick saliva often occurs at the site of a tick bite. This rash can be confused with the rash of Lyme disease. Allergic reactions to tick saliva usually occur within hours to a few days after the tick bite, usually do not expand, and disappear within a few days. Erythema migrans persists longer, but usually subsides within 3-4 weeks.

The presentation of erythema migrans in Stage 1
Stage two (dissemination stage) occurs days to weeks following infection. At this stage the spirochetes spread hematogenously to additional body tissues. One or more of the following symptoms and signs may be noted:
fatigue
chills and fever
headache
muscle and joint pain
swollen lymph nodes
secondary annular skin lesions
Stage three (persistent infection). Some symptoms and signs of Lyme disease may not appear until weeks, months, or years after a tick bite. Stage three typically involves intermittent episodes of joint pain. Common clinical manifestations at this stage may include meningitis, Bell's palsy, cardiac involvement, and migratory pain to joints, tendons, muscle and bone.
Arthritis is most likely to appear as brief bouts of pain and swelling, usually in one or more large joints, especially the knees.
Nervous system abnormalities can include numbness, pain, Bell's palsy (paralysis of the facial muscles, usually on one side), and meningitis (fever, stiff neck, and severe headache).
Less frequently, irregularities of the heart rhythm occur.
In a minority of individuals (11%) the development of chronic Lyme arthritis may lead to erosion of cartilage and/or bone. Other clinical manifestations associated with stage three Lyme disease include neurologic complications such as depression, disturbances in memory, mood, or sleep patterns, and sensations of numbness and tingling in the hands or feet.
Diagnosis of Lyme disease
Lyme disease is often difficult to diagnose because its symptoms and signs mimic those of so many other diseases. The fever, muscle aches, and fatigue of Lyme disease can easily be mistaken for viral infections, such as influenza or infectious mononucleosis. Joint pain can be mistaken for other types of arthritis, such as rheumatoid arthritis, and neurologic signs can mimic those caused by other conditions, such as multiple sclerosis. At the same time, other types of arthritis or neurologic diseases can be misdiagnosed as Lyme disease.
The clinical diagnosis of Lyme disease is usually based on history of possible exposure to ticks, especially in areas where Lyme disease is known to occur and a combination of symptoms and signs of infection. Serodiagnosis to detect anti-borrelia antibodies is not useful until in later stages of illness. Serologic testing may, however, provide valuable supportive diagnostic information in patients with endemic exposure and/or clinical findings that suggest late stage or disseminated Lyme disease.
When serologic testing is indicated, CDC recommends testing first with an enzyme-linked immunosorbent assay (ELISA) or an indirect fluorescent antibody (IFA) test, followed by a more specific Western immunoblot (WB) test to corroborate equivocal or positive results obtained with the first test. None of these tests is useful in the diagnosis of early stages of Lyme disease since a primary serum immune response is just beginning. Furthermore, these tests are associated with a high degree of cross-reactivity, since sera from patients with Rocky Mountain spotted fever, relapsing fever, mononucleosis, syphilis, and rheumatoid arthritis often test positive for Lyme disease.
Patients with early disseminated or late-stage disease usually have strong serological reactivity. Antibodies may persist for months or years following successfully treated or untreated infection. Thus, seroreactivity alone cannot be used as a marker of active disease.
Neither a positive serologic activity nor a history of previous Lyme disease assures that an individual has protective immunity. Repeated infection with B. burgdorferi has been documented.
B. burgdorferi can be cultured from 80% or more of biopsy specimens taken from early erythema migrans lesions. However, the diagnostic value of this procedure is limited because of the need for special bacteriologic media (BSK medium) and protracted observation of cultures.

Lyme disease is spread by the bite of ticks of the genus Ixodes that are infected with Borrelia burgdorferi. Ixodes, commonly known as the deer tick (or bear tick), normally feeds on the white-footed mouse, the white-tailed deer, and certain other mammals. It is responsible for transmitting the spirochetes to humans in the northeastern and north-central United States. On the Pacific Coast, the bacteria are transmitted to humans by the western black-legged tick, and in the southeastern states by the related black-legged tick.

Distribution of Ixodes ticks that transmit Lyme disease in the U.S. CDC.
Ixodes ticks are much smaller than common dog and cattle ticks. In their larval and nymphal stages, they are no bigger than a pinhead. Adult ticks are slightly larger. The tick nymphs, which are most likely to feed on a person and are rarely noticed because of their small size (less than 2 mm), are usually involved in the transmission of the disease.

Ixodes ticks: larva, nymph, adult male, adult female. CDC.
Spirochete prevalence in adult Ixodes ticks is highly variable depending on geographic location. It was shown to be present in approximately 35% of ticks in the Baraboo Hills northwest of Madison, Wisconsin, while in regions of California, 2% prevalence has been reported, and in regions of New York, 50% has been reported.
For Lyme disease to exist in an area, at least three closely interrelated elements must be present in nature: the Lyme disease bacteria, Borrelia burgdorferi,ticks that can transmit them, and mammals (such as mice and deer) to provide food for the ticks in their various life stages.
The tick life cycle consists of three distinctive stages: larvae, nymphs, and adults. A blood meal is required for ticks to molt from the larvae stage to the nymph stage and from the nymph stage to the adult stage. The tick larvae and nymphs typically become infected with Borrelia burgdorferi when they feed on infected small animals, particularly the white-footed mouse. The bacteria remain in the tick as it changes from larva to nymph or from nymph to adult. Infected nymphs and adult ticks then bite and transmit the bacteria to other small rodents, other animals, and humans, all in the course of their normal feeding behavior. Adult ticks preferentially feed on the white-tailed deer, which thereby becomes an important reservoir in regions of infestation. The tick life cycle takes two years to complete (see diagram below).

Lyme disease occurs in domestic animals, as well. In dogs, the disease usually presents as arthritis. Domestic animals can carry infected ticks into areas where humans live, but whether pet owners are more likely than others to get Lyme disease is not known.
The symptoms of Lyme disease in humans occur in three stages.
Stage one (early infection). The early stage of Lyme disease is often characterized by a distinctive, expanding red rash that usually develops at the site of the tick bite. This rash, known as erythema migrans, is seen in 60-80% of infected individuals (it is important to remember that the converse is true: no rash is ever observed in 20-40% of the cases). Spirochetes can be isolated from the leading edge of the rash. Erythema migrans is a red circular patch that appears usually 3 days to 1 month following the bite of the tick. The patch then expands, often to a large size and develops a characteristic "bull's eye" appearance. However, not all rashes that occur at the site of a tick bite are due to Lyme disease. An allergic reaction to tick saliva often occurs at the site of a tick bite. This rash can be confused with the rash of Lyme disease. Allergic reactions to tick saliva usually occur within hours to a few days after the tick bite, usually do not expand, and disappear within a few days. Erythema migrans persists longer, but usually subsides within 3-4 weeks.

The presentation of erythema migrans in Stage 1
Stage two (dissemination stage) occurs days to weeks following infection. At this stage the spirochetes spread hematogenously to additional body tissues. One or more of the following symptoms and signs may be noted:
fatigue
chills and fever
headache
muscle and joint pain
swollen lymph nodes
secondary annular skin lesions
Stage three (persistent infection). Some symptoms and signs of Lyme disease may not appear until weeks, months, or years after a tick bite. Stage three typically involves intermittent episodes of joint pain. Common clinical manifestations at this stage may include meningitis, Bell's palsy, cardiac involvement, and migratory pain to joints, tendons, muscle and bone.
Arthritis is most likely to appear as brief bouts of pain and swelling, usually in one or more large joints, especially the knees.
Nervous system abnormalities can include numbness, pain, Bell's palsy (paralysis of the facial muscles, usually on one side), and meningitis (fever, stiff neck, and severe headache).
Less frequently, irregularities of the heart rhythm occur.
In a minority of individuals (11%) the development of chronic Lyme arthritis may lead to erosion of cartilage and/or bone. Other clinical manifestations associated with stage three Lyme disease include neurologic complications such as depression, disturbances in memory, mood, or sleep patterns, and sensations of numbness and tingling in the hands or feet.
Lyme disease mimics other diseases and pathologies and is highly variable in its presentation. In some persons the rash never forms; in some, the first and only sign of Lyme disease is arthritis, and in others, nervous system problems are the only evidence of Lyme disease. There is an increasing and alarming number of reports of neuropsychiatric effects associated with Lyme Disease.
Lyme disease is often difficult to diagnose because its symptoms and signs mimic those of so many other diseases. The fever, muscle aches, and fatigue of Lyme disease can easily be mistaken for viral infections, such as influenza or infectious mononucleosis. Joint pain can be mistaken for other types of arthritis, such as rheumatoid arthritis, and neurologic signs can mimic those caused by other conditions, such as multiple sclerosis. At the same time, other types of arthritis or neurologic diseases can be misdiagnosed as Lyme disease.
The clinical diagnosis of Lyme disease is usually based on history of possible exposure to ticks, especially in areas where Lyme disease is known to occur and a combination of symptoms and signs of infection. Serodiagnosis to detect anti-borrelia antibodies is not useful until in later stages of illness. Serologic testing may, however, provide valuable supportive diagnostic information in patients with endemic exposure and/or clinical findings that suggest late stage or disseminated Lyme disease.
When serologic testing is indicated, CDC recommends testing first with an enzyme-linked immunosorbent assay (ELISA) or an indirect fluorescent antibody (IFA) test, followed by a more specific Western immunoblot (WB) test to corroborate equivocal or positive results obtained with the first test. None of these tests is useful in the diagnosis of early stages of Lyme disease since a primary serum immune response is just beginning. Furthermore, these tests are associated with a high degree of cross-reactivity, since sera from patients with Rocky Mountain spotted fever, relapsing fever, mononucleosis, syphilis, and rheumatoid arthritis often test positive for Lyme disease.
Patients with early disseminated or late-stage disease usually have strong serological reactivity. Antibodies may persist for months or years following successfully treated or untreated infection. Thus, seroreactivity alone cannot be used as a marker of active disease.
Neither a positive serologic activity nor a history of previous Lyme disease assures that an individual has protective immunity. Repeated infection with B. burgdorferi has been documented.
B. burgdorferi can be cultured from 80% or more of biopsy specimens taken from early erythema migrans lesions. However, the diagnostic value of this procedure is limited because of the need for special bacteriologic media (BSK medium) and protracted observation of cultures.
The polymerase chain reaction (PCR) has been used to amplify genomic DNA ofB. burgdorferi in skin, blood, cerebrospinal fluid, and synovial fluid, but PCR has not been standardized for routine diagnosis of Lyme disease.
Treatment of Lyme disease
Since the diagnosis of Lyme disease is based primarily on clinical findings, it is often appropriate to treat patients with early disease solely on the basis of objective signs and a known exposure.
Several antibiotics are effective in the treatment of Lyme disease. The present drug of choice is doxycycline, a semisynthetic derivative of tetracycline. Even patients who are treated in later stages of the disease respond well to antibiotics. In a few patients who are treated for Lyme disease, symptoms of persisting infection may continue or recur, making additional antibiotic treatment necessary. Varying degrees of permanent damage to joints or the nervous system can develop in patients with late chronic Lyme disease. Typically these are patients in whom Lyme disease was unrecognized in the early stages or for whom the initial treatment was unsuccessful.
Prevention
Removing leaves and clearing brush and tall grass around houses and at the edges of gardens may reduce the numbers of ticks that transmit Lyme disease. A relationship has been observed between the abundance of deer and the abundance of deer ticks in some parts United States. Reducing and managing deer populations in geographic areas where Lyme disease occurs may reduce tick abundance.
CDC recommends the following for personal protection from tick bites and Lyme disease:
Avoid tick-infested areas, especially in May, June, and July.
Wear light-colored clothing so that ticks can be spotted more easily. Tuck pant legs into socks or boots and shirt into pants or ape the area where pants and socks meet so that ticks cannot crawl under clothing.
Spray insect repellent containing DEET on clothes and on exposed skin other than the face, or treat clothes (especially pants, socks, and shoes) with permethrin, which kills ticks on contact.
Wear a hat and a long-sleeved shirt for added protection.
Walk in the center of trails to avoid overhanging grass and brush.
After being outdoors, remove clothing and wash and dry it at a high temperature; inspect body carefully and remove attached ticks with tweezers, grasping the tick as close to the skin surface as possible and pulling straight back with a slow steady force; avoid crushing the tick's body. In some areas, ticks (saved in a sealed container) can be submitted to the local health department for identification.
Preventive antibiotic treatment with erythromycin or doxycycline to prevent Lyme disease after a known tick bite may be warranted.
Personal protective measures, such as repellent use and routine tick checks, are key components of primary prevention. Removing infected ticks within 48 hours of attachment can reduce the likelihood of transmission, and prompt antimicrobial prophylaxis of tick bites, although controversial, might be beneficial under certain circumstances. Exposure to ticks in yards, playgrounds and recreational areas can be reduced 50-90% through simple landscaping practices, such as removing brush and leaf litter or creating a buffer zone of wood chips or gravel between forest and lawn or recreational areas. Correctly timed applications of pesticides to yards once or twice a year can decrease the number of nymphal ticks 68-100%.
In addition to these interventions, several novel approaches to Lyme disease prevention are under investigation and may soon be available. These include bait boxes and "four-poster" devices that deliver acaricides to rodents and deer without harming them, and the use of biologic agents, such as fungi that killIxodes ticks.
Vaccines for Lyme disease
In 1998, the Food and Drug Administration licensed the LYMErixTM vaccine against Lyme disease for human use. LYMErixTM contains lipidated recombinant outer surface protein A (OspA) of Borrelia burgdorferi sensu stricto, the causative agent of Lyme disease in North America, adsorbed onto aluminum adjuvant. It was indicated for use in persons aged 15-70 years. Three doses of the vaccine are administered by intramuscular injection. The initial dose is followed by a second dose one month later and a third dose 12 months after the first. Vaccine administration should be timed so the second dose and the third dose are given several weeks before the beginning of the B. burgdorferi transmission season which usually begins in April.
The vaccine was targeted at persons at risk for exposure to infected vector ticks. This risk should be assessed by considering the regional distribution of the disease and the extent to which a person's activities place them in contact with ticks. A Lyme disease risk map (below) is available from CDC. Vaccination of persons with frequent or prolonged exposure to ticks in areas endemic for Lyme disease was touted to be an important preventive strategy. Recommendations for use of the LYMErixTM vaccine were developed by the Advisory Committee for Immunization Practices of the CDC.
In February, 2002, the manufacturer of the FDA-approved LYMErixTM vaccine withdrew it from the market, reportedly because of poor sales. However, several other effective preventive measures remain available to persons living in areas where the disease is endemic.

No comments:
Post a Comment