Salmonella and Salmonellosis (page 1)
(This chapter has 5 pages)
Salmonella is a Gram-negative facultative rod-shaped bacterium in the same proteobacterial family as Escherichia coli, the family Enterobacteriaceae,trivially known as "enteric" bacteria. Salmonella is nearly as well-studied as E. coli from a structural, biochemical and molecular point of view, and as poorly understood as E. coli from an ecological point of view. Salmonellae live in the intestinal tracts of warm and cold blooded animals. Some species are ubiquitous. Other species are specifically adapted to a particular host. In humans,Salmonella are the cause of two diseases called salmonellosis: enteric fever(typhoid), resulting from bacterial invasion of the bloodstream, and acute gastroenteritis, resulting from a foodborne infection/intoxication.

Figure 1. Salmonella typhi, the agent of typhoid. Gram stain. (CDC)

Figure 2. Flagellar stain of a Salmonella Typhi. Like E. coli, Salmonella are motile by means of peritrichous flagella. A close relative that causes enteric infections is the bacterium Shigella. Shigella is not motile, and therefore it can be differentiated from Salmonella on the bais of a motility test or a flagellar stain. (CDC)

Figure 3. Salmonella sp. after 24 hours growth on XLD agar. Xylose Lysine (XL) agar is used when trying to culture and isolate Gram-negative enteric bacilli. When XL agar is supplemented with sodium thiosulfate, ferric ammonium citrate, and sodium deoxycholate, it is then termed XLD agar, and is then an even more selective medium than XL alone. The presence of any black colored area indicates the deposition of hydrogen sulfide, (H2S) under alkaline conditions. (CDC)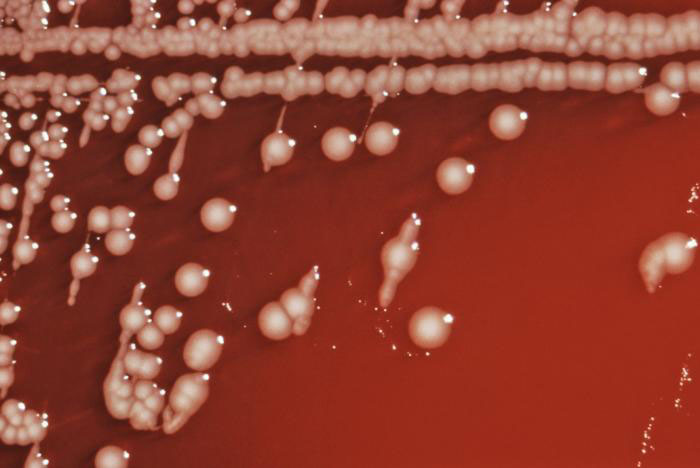
Figure 4. Colonial growth Salmonella choleraesuis subsp. arizonae bacteria grown on a blood agar culture plate. Also known as Salmonella Arizonae, it is a zoonotic bacterium that can infect humans, birds, reptiles, and other animals. (CDC)

Figure 5. Colonial growth pattern displayed by Salmonella Typhimurium cultured on a Hektoen enteric (HE) agar. S. Typhimurium colonies grown on HE agar are blue-green in color indcating that the bacterium does not ferment lactose However it does produce hydrogen sulfide, (H2S), as indicated by black deposits in the centers of the colonies. (CDC). HE agar is the medium designed for the isolation and recovery of fecal bacteria belonging to the family, Enterbacteriaceae. S.Typhimurium causes 25% of the 1.4 million salmonellosis infections a year in the United States. Most persons infected with Salmonella sp. develop diarrhea, fever, and abdominal cramps 12 - 72 hours after infection. The illness usually lasts 4 - 7 days, and most people recover without treatment. However, in some cases, the diarrhea may be so severe that the patient needs to be hospitalized.

Figure 6. Salmonella that has been cultured in a tetrathionate-enrichment broth, and stained using the direct fluorescent-antibody (FA) technique. Tetrathionate-enrichment broth contains bile salts, thereby inhibiting the growth of Gram-positive organisms, while the Gram-negative Salmonella sp., being an organism that possess the enzyme tetrathionate reductase, is able to break down tetrathionate, and grow uninhibited. (CDC)
END OF CHAPTER
(This chapter has 5 pages)
Salmonella is a Gram-negative facultative rod-shaped bacterium in the same proteobacterial family as Escherichia coli, the family Enterobacteriaceae,trivially known as "enteric" bacteria. Salmonella is nearly as well-studied as E. coli from a structural, biochemical and molecular point of view, and as poorly understood as E. coli from an ecological point of view. Salmonellae live in the intestinal tracts of warm and cold blooded animals. Some species are ubiquitous. Other species are specifically adapted to a particular host. In humans,Salmonella are the cause of two diseases called salmonellosis: enteric fever(typhoid), resulting from bacterial invasion of the bloodstream, and acute gastroenteritis, resulting from a foodborne infection/intoxication.
Discovery of the Typhoid Bacillus
At the beginning of the 19th century, typhoid was defined on the basis of clinical signs and symptoms and pathological (anatomical) changes. However, at this time, all sorts of enteric fevers were characterized as "typhoid".
In 1880s, the typhoid bacillus was first observed by Eberth in spleen sections and mesenteric lymph nodes from a patient who died from typhoid. Robert Koch confirmed a related finding by Gaffky and succeeded in cultivating the bacterium in 1881. But due to the lack of differential characters, separation of the typhoid bacillus from other enteric bacteria was uncertain.
In 1896, it was demonstrated that the serum from an animal immunized with the typhoid bacillus agglutinated (clumped) the typhoid bacterial cells, and it was shown that the serum of patients afflicted with typhoid likewise agglutinated the typhoid bacillus. Serodiagnosis of typhoid was thus made possible by 1896.

Figure 1. Salmonella typhi, the agent of typhoid. Gram stain. (CDC)
Salmonella Nomenclature
The genus Salmonella is a member of the family Enterobacteriaceae, It is composed of bacteria related to each other both phenotypically and genotypically. Salmonella DNA base composition is 50-52 mol% G+C, similar to that of Escherichia, Shigella, and Citrobacter. The bacteria of the genusSalmonella are also related to each other by DNA sequence. The genera with DNA most closely related to Salmonella are Escherichia, Shigella, andCitrobacter. Similar relationships were found by numerical taxonomy and 16S ssRNA analysis.
Salmonella nomenclature has been controversial since the original taxonomy of the genus was not based on DNA relatedness, rather names were given according to clinical considerations, e.g., Salmonella typhi, Salmonella cholerae-suis, Salmonella abortus-ovis, and so on. When serological analysis was adopted into the Kauffmann-White scheme in 1946, a Salmonella species was defined as "a group of related fermentation phage-type" with the result that eachSalmonella serovar was considered as a species. Since the host-specificity suggested by some of these earlier names does not exist (e.g., S. typhi-murium, S. cholerae-suis are in fact ubiquitous), names derived from the geographical origin of the first isolated strain of the newly discovered serovars were next chosen, e.g., S. london, S. panama, S. stanleyville.
Susequently it was found that all Salmonella serovars form a single DNA hybridization group, i.e., a single species composed of seven subspecies, and thenomenclature had to be adapted. To avoid confusion with the familiar names of serovars, the species name Salmonella enterica was proposed with the following names for the subspecies:
enterica I
salamae II
arizonae IIIa
diarizonae IIIb
houtenae IV
bongori V
indica VI
Each subspecies contains various serovars defined by a characteristic antigenic formula.
enterica I
salamae II
arizonae IIIa
diarizonae IIIb
houtenae IV
bongori V
indica VI
Each subspecies contains various serovars defined by a characteristic antigenic formula.
Since this formal Latin nomenclature may not be clearly understood by physicians and epidemiologists, who are the most familiar with the names given to the most common serovars, the common serovars names are kept for subspecies I strains, which represent more than 99.5% of the Salmonella strains isolated from humans and other warm-blooded animals. The vernacular terminology seems preferred in medical practice, e.g., Salmonella ser. Typhimurium (not italicized) or shorter Salmonella (or S.) Typhimurium.
Salmonella and Salmonellosis (page 2)
(This chapter has 5 pages)
© Kenneth Todar, PhD
(This chapter has 5 pages)
© Kenneth Todar, PhD
Antigenic Structure
As with all Enterobacteriaceae, the genus Salmonella has three kinds of major antigens with diagnostic or identifying applications: somatic, surface, and flagellar.
Somatic (O) or Cell Wall Antigens
Somatic antigens are heat stable and alcohol resistant. Cross-absorption studies individualize a large number of antigenic factors, 67 of which are used for serological identification. O factors labeled with the same number are closely related, although not always antigenically identical.
Somatic antigens are heat stable and alcohol resistant. Cross-absorption studies individualize a large number of antigenic factors, 67 of which are used for serological identification. O factors labeled with the same number are closely related, although not always antigenically identical.
Surface (Envelope) Antigens
Surface antigens, commonly observed in other genera of enteric bacteria (e.g.,Escherichia coli and Klebsiella), may be found in some Salmonella serovars. Surface antigens in Salmonella may mask O antigens, and the bacteria will not be agglutinated with O antisera. One specific surface antigen is well known: the Vi antigen. The Vi antigen occurs in only three Salmonella serovars (out of about 2,200): Typhi, Paratyphi C, and Dublin. Strains of these three serovars may or may not have the Vi antigen.
Surface antigens, commonly observed in other genera of enteric bacteria (e.g.,Escherichia coli and Klebsiella), may be found in some Salmonella serovars. Surface antigens in Salmonella may mask O antigens, and the bacteria will not be agglutinated with O antisera. One specific surface antigen is well known: the Vi antigen. The Vi antigen occurs in only three Salmonella serovars (out of about 2,200): Typhi, Paratyphi C, and Dublin. Strains of these three serovars may or may not have the Vi antigen.
Flagellar (H) Antigens
Flagellar antigens are heat-labile proteins. Mixing salmonella cells with flagella-specific antisera gives a characteristic pattern of agglutination (bacteria are loosely attached to each other by their flagella and can be dissociated by shaking). Also, antiflagellar antibodies can immobilize bacteria with corresponding H antigens.
Flagellar antigens are heat-labile proteins. Mixing salmonella cells with flagella-specific antisera gives a characteristic pattern of agglutination (bacteria are loosely attached to each other by their flagella and can be dissociated by shaking). Also, antiflagellar antibodies can immobilize bacteria with corresponding H antigens.
A few Salmonella entericaserovars (e.g., Enteritidis, Typhi) produce flagella which always have the same antigenic specificity. Such an H antigen is then called monophasic. Most Salmonella serovars, however, can alternatively produce flagella with two different H antigenic specificities. The H antigen is then called diphasic. For example, Typhimurium cells can produce flagella with either antigen i or antigen 1,2. If a clone is derived from a bacterial cell with H antigen i, it will consist of bacteria with i flagellar antigen. However, at a frequency of 10-3- 10-5, bacterial cells with 1,2 flagellar antigen pattern will appear in this clone.

Figure 2. Flagellar stain of a Salmonella Typhi. Like E. coli, Salmonella are motile by means of peritrichous flagella. A close relative that causes enteric infections is the bacterium Shigella. Shigella is not motile, and therefore it can be differentiated from Salmonella on the bais of a motility test or a flagellar stain. (CDC)
Habitats
The principal habitat of the salmonellae is the intestinal tract of humans and animals. Salmonella serovars can be found predominantly in one particular host, can be ubiquitous, or can have an unknown habitat. Typhi and Paratyphi A are strictly human serovars that may cause grave diseases often associated with invasion of the bloodstream. Salmonellosis in these cases is transmitted through fecal contamination of water or food. Gallinarum, Abortusovis, and Typhisuis are, respectively, avian, ovine, and porcine Salmonella serovars. Such host-adapted serovars cannot grow on minimal medium without growth factors (contrary to the ubiquitous Salmonella serovars).
Ubiquitous (non-host-adapted) Salmonella serovars (e.g., Typhimurium) cause very diverse clinical symptoms, from asymptomatic infection to serious typhoid-like syndromes in infants or certain highly susceptible animals (mice). In human adults, ubiquitous Salmonella organisms are mostly responsible for foodborne toxic infections.
The pathogenic role of a number of Salmonella serovars is unknown. This is especially the case with serovars from subspecies II to VI. A number of these serovars have been isolated rarely (some only once) during a systematic search in cold-blooded animals.
Salmonella in the Natural Environment
Salmonellae are disseminated in the natural environment (water, soil, sometimes plants used as food) through human or animal excretion. Humans and animals (either wild or domesticated) can excrete Salmonella either when clinically diseased or after having had salmonellosis, if they remain carriers.Salmonella organisms do not seem to multiply significantly in the natural environment (out of digestive tracts), but they can survive several weeks in water and several years in soil if conditions of temperature, humidity, and pH are favorable.
Salmonellae are disseminated in the natural environment (water, soil, sometimes plants used as food) through human or animal excretion. Humans and animals (either wild or domesticated) can excrete Salmonella either when clinically diseased or after having had salmonellosis, if they remain carriers.Salmonella organisms do not seem to multiply significantly in the natural environment (out of digestive tracts), but they can survive several weeks in water and several years in soil if conditions of temperature, humidity, and pH are favorable.
Isolation and Identification of Salmonella
A number of plating media have been devised for the isolation of Salmonella.Some media are differential and nonselective, i.e., they contain lactose with a pH indicator, but do not contain any inhibitor for non salmonellae (e.g., bromocresol purple lactose agar). Other media are differential and slightly selective, i.e., in addition to lactose and a pH indicator, they contain an inhibitor for nonenterics (e.g., MacConkey agar and eosin-methylene blue agar).
The most commonly used media selective for Salmonella are SS agar, bismuth sulfite agar, Hektoen enteric (HE) medium, brilliant green agar and xylose-lisine-deoxycholate (XLD) agar. All these media contain both selective and differential ingredients and they are commercially available.

Figure 3. Salmonella sp. after 24 hours growth on XLD agar. Xylose Lysine (XL) agar is used when trying to culture and isolate Gram-negative enteric bacilli. When XL agar is supplemented with sodium thiosulfate, ferric ammonium citrate, and sodium deoxycholate, it is then termed XLD agar, and is then an even more selective medium than XL alone. The presence of any black colored area indicates the deposition of hydrogen sulfide, (H2S) under alkaline conditions. (CDC)
Media used for Salmonella identification are those used for identification of allEnterobacteriaceae. Most Salmonella strains are motile with peritrichous flagella, however, nonmotile variants may occur occasionally. Most strains grow on nutrient agar as smooth colonies, 2-4 mm in diameter. Most strains are prototrophs, not requiring any growth factors. However, auxotrophic strains do occur, especially in host-adapted serovars such as Typhi and Paratyphi A.

Figure 4. Colonial growth Salmonella choleraesuis subsp. arizonae bacteria grown on a blood agar culture plate. Also known as Salmonella Arizonae, it is a zoonotic bacterium that can infect humans, birds, reptiles, and other animals. (CDC)
Table 1. Characteristics shared by most Salmonella strains belonging to subspecies I
Motile, Gram-negative bacteria
Lactose negative; acid and gas from glucose, mannitol, maltose, and sorbitol; no Acid from adonitol, sucrose, salicin, lactose
ONPG test negative (lactose negative)
Indole test negative
Methyl red test positive
Voges-Proskauer test negative
Citrate positive (growth on Simmon's citrate agar)
Lysine decarboxylase positive
Urease negative
Ornithine decarboxylase positive
H2S produced from thiosulfate
Do not grow with KCN
Phenylalanine and tryptophan deaminase negative
Gelatin hydrolysis negative
Lactose negative; acid and gas from glucose, mannitol, maltose, and sorbitol; no Acid from adonitol, sucrose, salicin, lactose
ONPG test negative (lactose negative)
Indole test negative
Methyl red test positive
Voges-Proskauer test negative
Citrate positive (growth on Simmon's citrate agar)
Lysine decarboxylase positive
Urease negative
Ornithine decarboxylase positive
H2S produced from thiosulfate
Do not grow with KCN
Phenylalanine and tryptophan deaminase negative
Gelatin hydrolysis negative

Figure 5. Colonial growth pattern displayed by Salmonella Typhimurium cultured on a Hektoen enteric (HE) agar. S. Typhimurium colonies grown on HE agar are blue-green in color indcating that the bacterium does not ferment lactose However it does produce hydrogen sulfide, (H2S), as indicated by black deposits in the centers of the colonies. (CDC). HE agar is the medium designed for the isolation and recovery of fecal bacteria belonging to the family, Enterbacteriaceae. S.Typhimurium causes 25% of the 1.4 million salmonellosis infections a year in the United States. Most persons infected with Salmonella sp. develop diarrhea, fever, and abdominal cramps 12 - 72 hours after infection. The illness usually lasts 4 - 7 days, and most people recover without treatment. However, in some cases, the diarrhea may be so severe that the patient needs to be hospitalized.
Genetics of Salmonella
The genetic map of the Salmonella Typhimurium strain LT2 is not very different from that of Escherichia coli K-12. The F plasmid can be transferred to Typhimurium, and an Hfr strain of Typhimurium may subsequently be selected. Conjugative chromosomal transfer may occur from Typhimurium Hfr to E. coli or from E. coli Hfr to Typhimurium. Chromosomal genes responsible for O, Vi, and H antigens can be transferred from Salmonella to Escherichia.
Also, Salmonella may harbor temperate phages and plasmids. Plasmids inSalmonella may code for antibiotic resistance (resistance plasmids are frequent due to the selective pressure of extensive antibiotic therapy), bacteriocins, metabolic characteristics such as lactose or sucrose fermentation, or antigenic changes of O antigen.
Pathogenesis of Salmomella Infections in Humans
Salmonella infections in humans vary with the serovar, the strain, the infectious dose, the nature of the contaminated food, and the host status. Certain serovars are highly pathogenic for humans; the virulence of more rare serovars is unknown. Strains of the same serovar are also known to differ in their pathogenicity. An oral dose of at least 105Salmonella Typhi cells are needed to cause typhoid in 50% of human volunteers, whereas at least 109 S.Typhimurium cells (oral dose) are needed to cause symptoms of a toxic infection. Infants, immunosuppressed patients, and those affected with blood disease are more susceptible to Salmonella infection than healthy adults.
In the pathogenesis of typhoid the bacteria enter the human digestive tract, penetrate the intestinal mucosa (causing no lesion), and are stopped in the mesenteric lymph nodes. There, bacterial multiplication occurs, and part of the bacterial population lyses. From the mesenteric lymph nodes, viable bacteria and LPS (endotoxin) may be released into the bloodstream resulting in septicemia Release of endotoxin is responsible for cardiovascular �collapsus and tuphos� (a stuporous state�origin of the name typhoid) due to action on the ventriculus neurovegetative centers.
Salmonella excretion by human patients may continue long after clinical cure. Asymptomatic carriers are potentially dangerous when unnoticed. About 5% of patients clinically cured from typhoid remain carriers for months or even years. Antibiotics are usually ineffective on Salmonella carriage (even if salmonellae are susceptible to them) because the site of carriage may not allow penetration by the antibiotic.
Salmonellae survive sewage treatments if suitable germicides are not used in sewage processing. In a typical cycle of typhoid, sewage from a community is directed to a sewage plant. Effluent from the sewage plant passes into a coastal river where edible shellfish (mussels, oysters) live. Shellfish concentrate bacteria as they filter several liters of water per hour. Ingestion by humans of these seafoods (uncooked or superficially cooked) may cause typhoid or other salmonellosis. Salmonellae do not colonize or multiply in contaminated shellfish.
Typhoid is strictly a human disease.The incidence of human disease decreases when the level of development of a country increases (i.e., controlled water sewage systems, pasteurization of milk and dairy products). Where these hygienic conditions are missing, the probability of fecal contamination of water and food remains high and so is the incidence of typhoid.
Foodborne Salmonella toxic infections are caused by ubiquitous Salmonellaserovars (e.g., Typhimurium). About 12-24 hours following ingestion of contaminated food (containing a sufficient number of Salmonella), symptoms appear (diarrhea, vomiting, fever) and last 2-5 days. Spontaneous cure usually occurs.
Salmonella may be associated with all kinds of food. Contamination of meat (cattle, pigs, goats, chicken, etc.) may originate from animal salmonellosis, but most often it results from contamination of muscles with the intestinal contents during evisceration of animals, washing, and transportation of carcasses. Surface contamination of meat is usually of little consequence, as proper cooking will sterilize it (although handling of contaminated meat may result in contamination of hands, tables, kitchenware, towels, other foods, etc.). However, when contaminated meat is ground, multiplication of Salmonella may occur within the ground meat and if cooking is superficial, ingestion of this highly contaminated food may produce a Salmonellainfection. Infection may follow ingestion of any food that supports multiplication of Salmonella such as eggs, cream, mayonnaise, creamed foods, etc.), as a large number of ingested salmonellae are needed to give symptoms. Prevention of Salmonella toxic infection relies on avoiding contamination (improvement of hygiene), preventing multiplication of Salmonella in food (constant storage of food at 4°C), and use of pasteurized and sterilized milk and milk products. Vegetables and fruits may carry Salmonella when contaminated with fertilizers of fecal origin, or when washed with polluted water.
The incidence of foodborne Salmonella infection/toxication remains reletavely high in developed countries because of commercially prepared food or ingredients for food. Any contamination of commercially prepared food will result in a large-scale infection. In underdeveloped countries, foodborne Salmonellaintoxications are less spectacular because of the smaller number of individuals simultaneously infected, but also because the bacteriological diagnosis ofSalmonella toxic infection may not be available. However, the incidence ofSalmonella carriage in underdeveloped countries is known to be high.
Salmonella epidemics may occur among infants in pediatric wards. The frequency and gravity of these epidemics are affected by hygienic conditions, malnutrition, and the excessive use of antibiotics that select for multiresistant strains.
Salmonella Enteritidis Infection
Egg-associated salmonellosis is an important public health problem in the United States and several European countries. Salmonella Enteritidis, can be inside perfectly normal-appearing eggs, and if the eggs are eaten raw or undercooked, the bacterium can cause illness. During the 1980s, illness related to contaminated eggs occurred mosy frequently in the northeastern United States, but now illness caused by S. Enteritidis is increasing in other parts of the country as well.
Egg-associated salmonellosis is an important public health problem in the United States and several European countries. Salmonella Enteritidis, can be inside perfectly normal-appearing eggs, and if the eggs are eaten raw or undercooked, the bacterium can cause illness. During the 1980s, illness related to contaminated eggs occurred mosy frequently in the northeastern United States, but now illness caused by S. Enteritidis is increasing in other parts of the country as well.
Unlike eggborne salmonellosis of past decades, the current epidemic is due to intact and disinfected grade A eggs. Salmonella Enteritidis silently infects the ovaries of healthy appearing hens and contaminates the eggs before the shells are formed. Most types of Salmonella live in the intestinal tracts of animals and birds and are transmitted to humans by contaminated foods of animal origin. Stringent procedures for cleaning and inspecting eggs were implemented in the 1970s and have made salmonellosis caused by external fecal contamination of egg shells extremely rare. However, unlike eggborne salmonellosis of past decades, the current epidemic is due to intact and disinfected grade A eggs. The reason for this is that Salmonella Enteritidis silently infects the ovaries of hens and contaminates the eggs before the shells are formed.
Although most infected hens have been found in the northeastern United States, the infection also occurs in hens in other areas of the country. In the Northeast, approximately one in 10,000 eggs may be internally contaminated. In other parts of the United States, contaminated eggs appear less common. Only a small number of hens seem to be infected at any given time, and an infected hen can lay many normal eggs while only occasionally laying an egg contaminated with Salmonella Enteritidis.
A person infected with the Salmonella Enteritidis usually has fever, abdominal cramps, and diarrhea beginning 12 to 72 hours after consuming a contaminated food or beverage. The illness usually lasts 4 to 7 days, and most persons recover without antibiotic treatment. However, the diarrhea can be severe, and the person may be ill enough to require hospitalization. The elderly, infants, and those with impaired immune systems (including HIV) may have a more severe illness. In these patients, the infection may spread from the intestines to the bloodstream, and then to other body sites and can cause death unless the person is treated promptly with antibiotics.
Exotoxins
Salmonella strains may produce a thermolabile enterotoxin that bears a limited relatedness to cholera toxin both structurally and antigenically. This enterotoxin causes water secretion in rat ileal loop and is recognized by antibodies against both cholera toxin and the thermolabile enterotoxin (LT) of enterotoxinogenic E. coli, but it does not bind in vitro to ganglioside GM1 (the receptor for E. coli LT and cholera ctx). Additionally, a cytotoxin that inhibits protein synthesis and is immunologically distinct from Shiga toxin has been demonstrated. Both of these toxins are presumed to play a role in the diarrheal symptoms of salmonellosis.
Antibiotic Susceptibility
During the last decade, antibiotic resistance and multiresistance of Salmonellaspp. have increased a great deal. The cause appears to be the increased and indiscriminate use of antibiotics in the treatment of humans and animals and the addition of growth-promoting antibiotics to the food of breeding animals. Plasmid-borne antibiotic resistance is very frequent among Salmonella strains involved in pediatric epidemics (e.g., Typhimurium, Panama, Wien, Infantis). Resistance to ampicillin, streptomycin, kanamycin, chloramphenicol, tetracycline, and sulfonamides is commonly observed. Colistin resistance has not yet been observed.
Until 1972, Typhi strains had remained susceptible to antibiotics, including chloramphenicol (the antibiotic most commonly used against typhoid) but in 1972, a widespread epidemic in Mexico was caused by a chloramphenicol-resistant strain of S. Typhi. Other chloramphenicol-resistant strains have since been isolated in India, Thailand, and Vietnam. Possible importation or appearance of chloramphenicol-resistance strains in the United States is a real threat. Salmonella strains should be systematically checked for antibiotic resistance to aid in the choice of an efficient drug when needed and to detect any change in antibiotic susceptibility of strains (either from animal or human source). Indiscriminate distribution and use of antibiotics should be discouraged.
Vaccination Against Typhoid Fever
Three types of typhoid vaccines are currently available for use in the United States: (1) an oral live-attenuated vaccine; (2) a parenteral heat-phenol-inactivated vaccine; (3) a newly licensed capsular polysaccharide vaccine for parenteral use. A fourth vaccine, an acetone-inactivated parenteral vaccine, is currently available only to the armed forces.
1. Live oral vaccines. Although oral killed vaccines are without efficacy, vaccines using living avirulent bacteria have shown promise. A galactose-epimeraseless mutant of Typhi has given very good results in a field trials. Mutants of Typhimurium that have given a good protection in animals include mutants lacking adenylate-cyclase and AMP receptor protein, and mutants auxotrophic for p-aminobenzoate and adenine.Typhi with the same mutations does not cause adverse reactions and is immunogenic in human.
The Live Oral Typhoid Vaccine should not be given to children younger than 6 years of age. It is given in four doses, 2 days apart, as needed for protection. The last dose should be given at least 1 week before travel to allow the vaccine time to work. A booster dose is needed every 5 years for people who remain at risk.
2. The parenteral heat-phenol-inactivated vaccine has been widely used for many years. In field trials involving a primary series of two doses of heat-phenol- inactivated typhoid vaccine, efficacy over the 2- to 3-year follow-up periods ranged from 51% to 77% . Efficacy for the acetone- inactivated parenteral vaccine, available only to the armed forces, ranges from 75% to 94%.
Since the inactivated vaccines contain the O antigen (endotoxin), local and general reactions occur. Vi antigen extracted following the methodology used for the meningococcal vaccine seems to avoid reactions to endotoxin.
The inactivated Typhoid Vaccine should not be given to children younger than 2 years of age. One dose provides protection. It should be given at least 2 weeks before travel to allow the vaccine time to work. A booster dose is needed every 2 years for people who remain at risk.
3. The newly licensed parenteral vaccine [Vi capsular polysaccharide (ViCPS)] is composed of purified Vi ("virulence") antigen, the capsular polysaccharide elaborated by S.Typhi isolated from blood cultures. In recent studies, one 25-ug injection of purified ViCPS produced seroconversion (i.e., at least a fourfold rise in antibody titers) in 93% of healthy U.S. adults. Two field trials in disease-endemic areas have demonstrated the efficacy of ViCPS in preventing typhoid fever. In one trial in Nepal, in which vaccine recipients were observed for 20 months, one dose of ViCPS among persons 5-44 years of age resulted in 74% fewer cases of typhoid fever. ViCPS has not been tested among children less than 1 year of age.
NOTE: No typhoid vaccine is 100% effective and is not a substitute for being careful about what you eat or drink.
Routine typhoid vaccination is not recommended in the United States, but typhoid vaccine is recommended for travellers to parts of the world where typhoid is common, people in close contact with a typhoid carriers, and laboratory workers who work with Salmonella Typhi bacteria.

Figure 6. Salmonella that has been cultured in a tetrathionate-enrichment broth, and stained using the direct fluorescent-antibody (FA) technique. Tetrathionate-enrichment broth contains bile salts, thereby inhibiting the growth of Gram-positive organisms, while the Gram-negative Salmonella sp., being an organism that possess the enzyme tetrathionate reductase, is able to break down tetrathionate, and grow uninhibited. (CDC)
For more information on salmonella infections please see
CDC Salmonellosis
CDC Salmonella enteritidis
CDC Salmonella Infection (salmonellosis) and Animals
CDC Typhoid General information
CDC Typhoid traveller's information
CDC Typhoid vaccine: What You Need to Know
FDA/CFSAN Bad Bug Book - Salmonella spp
MedlinePlus Enteric fever
NOVA | The Most Dangerous Woman in America (Typhoid Mary)
Typhoid and paratyphoid fever (UK)
Typhoid Fever Utah Health Dept
Typhoid fever NY Communicable Disease Fact Sheet
WHO Salmonella
WHO Typhoid fever
CDC Salmonellosis
CDC Salmonella enteritidis
CDC Salmonella Infection (salmonellosis) and Animals
CDC Typhoid General information
CDC Typhoid traveller's information
CDC Typhoid vaccine: What You Need to Know
FDA/CFSAN Bad Bug Book - Salmonella spp
MedlinePlus Enteric fever
NOVA | The Most Dangerous Woman in America (Typhoid Mary)
Typhoid and paratyphoid fever (UK)
Typhoid Fever Utah Health Dept
Typhoid fever NY Communicable Disease Fact Sheet
WHO Salmonella
WHO Typhoid fever
END OF CHAPTER
No comments:
Post a Comment